Recently, the enterprise Hyperway in Tianfu Life Science Park has brought good news, with significant R&D progress made in its self-developed analgesic drugs.
R&D Progress in Analgesics by Hyperway
Hyperway has built a sound innovative brain-penetrant drug development platform and focuses on the development of drugs for central nervous system diseases, with pain being one of the key areas in its strategic plan. In addition to the R&D pipeline of Nav1.8 inhibitor that has entered clinical study, Hyperway has also established another pipeline for cutting-edge, non-addictive analgesic targeted drugs. At present, there is no such targeted drug on the market globally, and the one with the fastest research progress has just entered Phase I clinical trials.
Abundantly expressed on small-diameter primary sensory neurons, this analgesic target is a newly identified pain-sensing pathway in recent years and can be activated by heat and chemical ligands. After activation, it shows greater permeability to calcium ions and can release CGRP, thus causing neurogenic inflammation. Studies have shown that the use of drugs with inhibitory effects on this target can significantly reduce mechanical and thermal hyperalgesia in mouse and rat models of inflammatory and neuropathic pain. Furthermore, other studies have shown that opioids like morphine strongly inhibit the activity of this target after activating the μ receptor, indicating that this novel pain target might be a peripheral analgesic target for opioid analgesics. In the future, this targeted drug is expected to replace opioid analgesics, addressing the international dilemma of the past century where strong analgesic effects cannot be achieved without addiction.
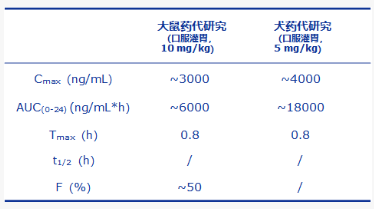
With keen comprehensive analysis capability of druggability, leading drug molecular design capability and rapid chemical synthesis capability, Hyperway has demonstrated a good pharmacokinetic profile of HBW-015-15 by oral administration in rats and dogs, and the bioavailability reaches 50% in rats (as shown in Table 1).
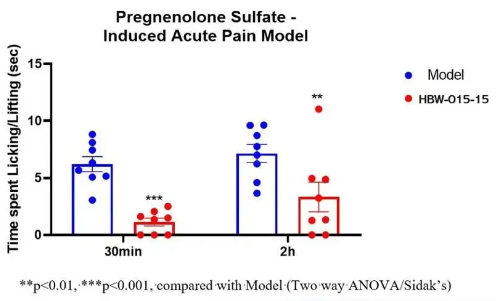
As seen in Figure 1, in the mouse model of acute pain induced by pregnenolone sulfate (PS), oral administration of HBW-015-15 at a dose of 5 mg/kg achieves significant analgesic effects within 30 minutes, demonstrating a rapid onset of action.
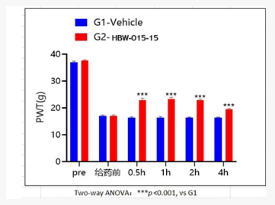
As seen in Figure 2, in the rat model of CCI (chronic constriction injury of the sciatic nerve) neuropathic pain, oral administration of HBW-015-15 at a dose of 5 mg/kg achieves significant analgesic effects within 30 minutes and the effects remain pronounced after 4 h.
Based on the existing study data, HBW-015-15 exhibits good inhibitory activity against targets and shows a favorable pharmacokinetic profile in pharmacokinetic studies of rats and dogs. It showcases an inhibition rate of less than 30% for 217 kinases at a concentration of 1 μM, with no inhibitory or agonistic effects on various ion channels, indicating high selectivity. Additionally, a 14-day toxicity study on rats has shown excellent safety profiles. In conclusion, HBW-015-15 fully meets the needs of further clinical development in terms of pharmacodynamics, pharmacokinetics and safety.
About Hyperway
Hyperway is a high-growth high-tech enterprise oriented to clinical needs and driven by source innovation, focusing on the R&D of targeted innovative drugs. The company has assembled an international and professional R&D management team, covering various professional fields in the whole chain of innovative drug R&D from target screening to registration and marketing. Hyperway boasts fast R&D speed and high-quality achievements: It has laid out more than ten innovative drug R&D pipelines, focusing on multiple hot fields such as tumors, pain and central nervous system diseases, and built a differentiated brain-penetrant drug development platform; it has applied for more than 30 invention patents and obtained 10 authorizations; it has determined 7 PCCs, 3 of which have entered the clinical study stage; many research results have been selected into prestigious international academic conferences such as ASCO and EHA, demonstrating Hyperway's solid independent innovation, industrial competitiveness and sustainable development strength in the pharmaceutical industry.
In future development, Hyperway will fully leverage its advantages in drug R&D, aiming to be internationally superior or a global pioneer, increase R&D investment, deepen cooperation, adopt an open, multi-level and multi-faceted cooperation mode, and seek external partners through multi-dimensional cooperation methods such as regional assignment of interest of innovative drugs, technical cooperation and technology transfer of innovative drugs and improved new drugs, so as to jointly develop high-quality and cost-effective drugs of China!



Park WeiChart