Recently, another two products of Chengdu Brilliant Pharmaceutical Co., Ltd. (hereinafter referred to as "Brilliant") in Chengdu Advanced Medical Science Center Park have been approved.
Two Major Products of Brilliant Pharmaceutical Obtain Approval
On May 21, according to the official website of the National Medical Products Administration (NMPA), Fluvoxamine Maleate Tablets and Epalrestat Tablets developed by Chengdu Brilliant Pharmaceutical Co., Ltd. were successfully approved.

From November 1, 2022, the administrative counterpart can log in to the legal person space of the online service hall to view the electronic certificate and print it according to relevant prompts.
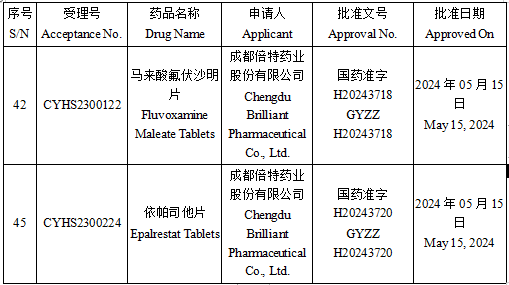
Fluvoxamine Maleate Tablets is a selective serotonin reuptake inhibitor mainly used for the treatment of depression and related symptoms, as well as symptoms of obsessive-compulsive disorder.
Epalrestat Tablets is a reversible non-competitive inhibitor of aldose reductase, mainly used in the treatment of diabetic neuropathy.
According to information obtained, as of 2024, 10 products of Brilliant Pharmaceutical have been approved for marketing, including Dapagliflozin Tablets, Gadobenate Dimeglumine Injection, Furosemide Oral Solution, Gadoteric Acid Meglumine Salt Injection, Empagliflozin tablets, Metaraminol Bitartrate Injection, Dexmedetomidine Hydrochloride Nasal Spray, Ioversol Injection, Epalrestat Tablets and Fluvoxamine Maleate Tablets, further enriching the company's product line.



Park WeiChart